Abstract
Background. Maintenance therapy in AML patients with measurable residual disease (MRD) remains challenging. Immunotherapy could be an effective way to induce durable disease control. This phase II study aims to show efficacy of priming the immune system, using an allogenic leukemia-derived dendritic cell vaccine (DCP-001), to induce an effective anti-leukemic response to control or kill remaining malignant cells (ADVANCE-II, Clintrials.gov: NCT03697707).
Methods. A total of 20 evaluable AML patients in first complete remission (CR1) with MRD positivity and ineligible for HSCT received 4 biweekly doses of 25e6 or 50e6 cells per intradermal vaccination (cells/vc) and 2 doses as boosts at week 14 and 18 of 10e6 cells/vc.
MRD, as primary endpoint, was assessed at baseline, week 14, 20 and 32 by flow cytometry and/or molecular analyses. Evaluation of secondary endpoints relapse-free (RFS) and overall survival (OS) was done at data cut-off (18th July 2022). Peripheral blood samples were taken before and during treatment for analysis of vaccine induced responses as measured by IFNγ ELISPOT and general immune profiles by flow cytometry using a 40-marker panel.
Results. Patients aged 34-79 (median 60y) had a cytogenetic risk status assessed of 4 better, 15 intermediate and 1 poor-risk, with mutations in NPM1 (n=10), CBFB-MYH11 (n=4), IDH2 (n=2), RUNX1-RUNX1T1 (n=2), TP53 (n=1) and CEBPA (n=1). All 20 patients have received 4 initial vaccinations, 17 patients received all booster vaccinations and 1 patient received one booster. No serious adverse events (AE) or severe AE (grade 3 or higher) related to the treatment have been reported. Related AEs are mainly injection site reactions, such as redness, warmth and swelling, occurring within 48 hours after intradermal administration.
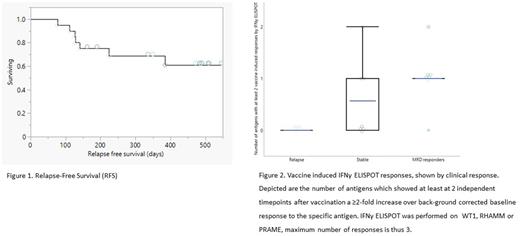
To date, median RFS and OS have not yet been reached (Figure 1), with a median follow up period of 15.4 (range 6.6- 16.8) months for the entire study population. Estimated RFS and OS at 6 months is 83.9% (range 64-94%) and 97% (range 79-99%), respectively. Estimated RFS and OS at 12 months is 70% (range 48-85%) and 87% (67-95%), respectively.
MRD response (conversion or >10-fold decrease of MRD) was observed in 7 patients: 5 patients converted to negative and 2 showed a MRD decrease. Responses were observed within 14-20 weeks and durable in 4 out of 5 patients, remaining MRD negative up to week 32. Seven patients remained in CR with stable MRD levels and 6 patients relapsed before week 32. Three patients with stable MRD became eligible for HSCT during study and were transplanted successfully. Immune responses were observed to tumor associated antigens expressed by DCP-001; WT1, RHAMM and PRAME, with specific vaccine induced responses (VIR) in the majority of patients (17/20) and highest number of VIR per antigen observed in patients with MRD responses (Figure 2). Differences in immune profiles at baseline were observed; relapsed patients had a high number of CD8RO+ cells, in particular CD8 CM. On the contrary, MRD responders had the highest level of CD8RA+ cells at baseline, with CD8 EMRA cells at the highest level. Patients with stable MRD had comparable, but slightly lower CD8RO+ levels than MRD responders.
Conclusion/discussion. Vaccination with DCP-001 resulted in durable MRD responses with five patients converting to MRD negativity, remaining relapse-free and alive during follow up. This translates in promising survival data with the median RFS for the full patient population not reached to date, at a median FU time of 15.4 months. Highest level of vaccine-induced T-cell responses to leukemia specific antigens were detected in MRD responders. Baseline levels of CD8 memory profiles were markedly different in MRD responders, which might be indicative that lower number of memory cells, and thus especially higher number of CD8 EMRA could reflect the ability to induce a tumor specific cytotoxic T-cell response after vaccination. Relapse on study was limited, with an estimated RFS at 6 months of 83.7%, which is higher than expected for this MRD+ AML patient population, which would be around 45% as shown for placebo-treated patients from a recent phase III study in a similar patient population (Quazar AML001). Immunotherapy with DCP-001 as AML maintenance therapy is safe and effective in triggering immune responses and converting patients from MRD positive to MRD negative, leading to promising relapse-free survival.
Disclosures
Van de Loosdrecht:Takeda: Honoraria; Amgen: Honoraria; Novartis: Honoraria; Celgene/BMS: Honoraria; Alexion: Research Funding. Cloos:Merus: Other: MRD assessments, Research Funding; Astellas: Speakers Bureau; Novartis: Consultancy, Other: MRD assessments, Research Funding; Janssen: Research Funding; Genentech: Research Funding; DC-One: Other: MRD assessments, Research Funding; Navigate: Patents & Royalties: Royalties for MRD analyses; Helsinn: Other: MRD assessments; Takeda: Research Funding. Platzbecker:Janssen: Honoraria; Silence Therapeutics: Honoraria; Jazz: Honoraria; Abbvie: Honoraria; BMS/Celgene: Honoraria; Novartis: Honoraria; Geron: Honoraria; Takeda: Honoraria. Holderried:Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; MSD: Speakers Bureau; Amgen: Speakers Bureau; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees. Giagounidis:BMS: Honoraria. van Zeeburg:Mendus AB: Current Employment. Rovers:Mendus AB: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal